|
As
with H , ,  ,
and ,
and  ,
we can establish the number of bonds between atoms in these molecules
by counting the net number of bonding electrons and dividing by
two, since we are accustomed to calling two bonding electrons a
"bond." Li ,
we can establish the number of bonds between atoms in these molecules
by counting the net number of bonding electrons and dividing by
two, since we are accustomed to calling two bonding electrons a
"bond." Li ,
found when lithium metal is vaporized, has a single bond. Be ,
found when lithium metal is vaporized, has a single bond. Be has no net bonding for the same reason He. has none, so vaporized
Be consists of single atoms. B
has no net bonding for the same reason He. has none, so vaporized
Be consists of single atoms. B has a single bond, C
has a single bond, C a double bond, N
a double bond, N and CO triple bonds, and 0
and CO triple bonds, and 0 a double bond because of the disruptive effect of the
a double bond because of the disruptive effect of the 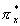 and and
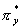 antibonding
electrons. F antibonding
electrons. F has only a single bond, and Ne
has only a single bond, and Ne has no bond at all, like He
has no bond at all, like He and Be
and Be . These
predictions about bond order from MO theory correspond quite well
with the observed bond lengths and bond energies, which are tabulated
below the filling diagrams. Bond lengths decrease and bond energies
increase with increasing bond order from B to N, and then reverse
their trends as the bond order decreases from N to Ne. N . These
predictions about bond order from MO theory correspond quite well
with the observed bond lengths and bond energies, which are tabulated
below the filling diagrams. Bond lengths decrease and bond energies
increase with increasing bond order from B to N, and then reverse
their trends as the bond order decreases from N to Ne. N and CO have the same bond structure and show similar bond lengths
and bond energies. The single-double- triple-double-single sequence
of bond orders for B
and CO have the same bond structure and show similar bond lengths
and bond energies. The single-double- triple-double-single sequence
of bond orders for B through F
through F is
entirely the consequence of the order of energies of the MO's as
diagramed at the left. If this sequence of energy levels had been
different-if all the bonding MO's had come before the antilionding,
for example-then the predictions of bonding in diatomic molecules
by MO theory would have been quite wrong. is
entirely the consequence of the order of energies of the MO's as
diagramed at the left. If this sequence of energy levels had been
different-if all the bonding MO's had come before the antilionding,
for example-then the predictions of bonding in diatomic molecules
by MO theory would have been quite wrong.
|
|