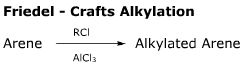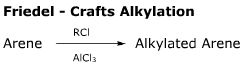
- This reaction is an example of electrophilic aromatic substitution.
- The product of this reaction is more reactive than the starting material (an activating substituent has been added) so product mixtures with multiple substitutions are formed.
- The 'R' group may rearrange during this reaction, giving yet more product mixture possibilities.
- For these reasons, this method is not preferred. Instead the Friedel-Crafts Acylation is used.
References;
- Branchaud B.P.; Blanchette H.S.; Tetrahedron Letters, 2002, Volume 43, Issue 3, 351-353
- Shiina I.; Suzuki M.; Yokoyama K.; Tetrahedron Lett., 2004, Volume 45, Issue 5, 965-967