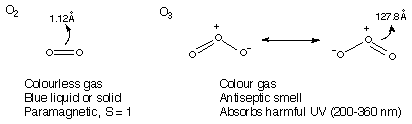
- m.p. -229 deg.C, b.p. -183 deg.C
- 16O 99.56% I = 0; 18O 0.4% ; 17O 0.04% I = 5/2
- 20.9% by volume of Earth's atmosphere.
- Most abundant element (46.6%) in Earth's crust.
- Essential for life.
- Two Allotropes: O2 and O3

* Ground state of O2 is a triplet S = 1
* Excited form of O2 with paired p* electrons (singlet) 1 Dg state
- red chemiluminesence 95 kJmol-1 above ground triplet state
- from H2O2 + OCl- ![]() Cl- + H2O + O2
Cl- + H2O + O2
- reactive, especially with organic compounds: 
MO Diagram for O2
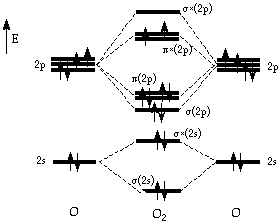
Figure 1: The molecular orbital diagram of dioxygen.