* Highly ionic oxides (Group 1 and 2) are basic.
* Highly covalent oxides (Groups 14-17) are acidic.
* The "amphoteric line" moves further to the right of Period as Groups are descended
* For elements of variable oxidation state, acidity increases with oxidation state.
cf N2O3 / N2O5 SO2 / SO3 Cl2O / ClO2 PbO / PbO2
Explanation:
* Concept of "Polarizing Power" explains the trends
Consider the reaction: EOn/2 + (6 + n/2)H2O ![]() E(H2O)6n+ + nOH-
E(H2O)6n+ + nOH-
~ En+ large (Group 1 and 2), E(H2O)6n+ ion stable, oxide is basic
~ En+ small, eg. B3+, Si4+, =>
Hydrolysis ![]() E(OH)n +
nH2O neutral
E(OH)n +
nH2O neutral
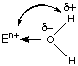
eg. Al(H2O)63+ is amphoteric Si(OH)4, or B(OH)3 are weakly acidic.
~ En+ very small, e.g. S6+, =? further hydrolysis, and strong acid properties.
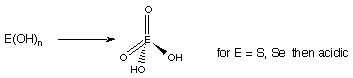
~ Explains why acidity increases with oxidation state.
* Alternative viewpoint, as En+ gets smaller preference for ![]()