Supplementary Material
Liquid ammonia: a non-aqueous
solvent
Despite low boiling point (-33.4oC), easy
to handle.
Solubilities,
relatively high dielectric constant (ammonia, eo=
26.7 @ -60oC;
water, eo
= 82 @ 18 deg.C).
=> ionic compounds can be soluble but the lower
eo
compared to water means that salt with highly charged,
non-polarisable anions such as carbonates, sulphates,
and phosphates are insoluble.
NH3 is more polarisable than H2O,
so salts with more polarisable anions are more soluble,
hence the solubility trends.
F- < Cl- < Br-
< I-
PO43- < SO42-
< OAc- < NO3
specific solvation: NH3 is a better a-donor
than H2O and ammine complexes are formed,
especially with the later transition (Ni2+,
Cu2+) and B metals (Ag+, Zn2+).
Hence higher solubilities for compounds of these
metals than those of the A-metals.
Self-ionization of ammonia
is much "weaker" than water.
2NH3 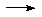 NH4+ + NH2-
k @ 223K ca. 10-30
Liquid ammonia will therefore tolerate very
strong bases such as
C5H5- which
would otherwise be hydrolysed in water.
Ammonia is kinetically
stabilized to reduction (but easily oxidized)
by many reagents, e.g., the reaction;
Na + NH3
NH4+ + NH2-
k @ 223K ca. 10-30
Liquid ammonia will therefore tolerate very
strong bases such as
C5H5- which
would otherwise be hydrolysed in water.
Ammonia is kinetically
stabilized to reduction (but easily oxidized)
by many reagents, e.g., the reaction;
Na + NH3 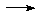 NaNH2 + H2(g)
is very favourable but slow in the absence
of a catalyst such as Fe3+.
Reactions and applications
Solvolysis: synthesis
of amides
OPCl3 + 6NH3
NaNH2 + H2(g)
is very favourable but slow in the absence
of a catalyst such as Fe3+.
Reactions and applications
Solvolysis: synthesis
of amides
OPCl3 + 6NH3 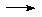 OP(NH2)3 + 3NH4Cl
SiCl4 + 8NH3
OP(NH2)3 + 3NH4Cl
SiCl4 + 8NH3 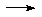 Si(NH2)4 + 4NH4Cl
Metatheses reactions:
solubility reversals
In water,
RCl + AgNO3
Si(NH2)4 + 4NH4Cl
Metatheses reactions:
solubility reversals
In water,
RCl + AgNO3 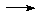 AgCl(ppt)
+ RNO3
In ammonia
AgCl + KNO3
AgCl(ppt)
+ RNO3
In ammonia
AgCl + KNO3 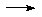 RCl(ppt)
+ AgNO3
Ba(NO3)2 + 2AgCl
RCl(ppt)
+ AgNO3
Ba(NO3)2 + 2AgCl 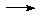 BaCl2(ppt) + 2AgNO3
Alkali metals in liquid ammonia
Sodamide as a base
Na + NH3
BaCl2(ppt) + 2AgNO3
Alkali metals in liquid ammonia
Sodamide as a base
Na + NH3 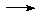 NaNH2 + H2(g)
NaNH2 + C5H6
NaNH2 + H2(g)
NaNH2 + C5H6 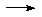 NaC5H5 + NH3
NaCp (useful reagent)
Solvated electron as a reducing agent (Birch
reduction),
many examples of compounds in very unusual low oxidation
states.
[Ni(CN)4]2- + Na/liq-NH3
NaC5H5 + NH3
NaCp (useful reagent)
Solvated electron as a reducing agent (Birch
reduction),
many examples of compounds in very unusual low oxidation
states.
[Ni(CN)4]2- + Na/liq-NH3 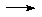 [Ni(CN)4]4-
Fe(CO)5 + Na/liq-NH3
[Ni(CN)4]4-
Fe(CO)5 + Na/liq-NH3 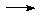 [Fe(CO)4]2-
Mo(CO)6 + Na/liq-NH3
[Fe(CO)4]2-
Mo(CO)6 + Na/liq-NH3 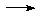 [Mo(CO)4]4-
[Pt(NH3)4]2+
+ Na/liq-NH3
[Mo(CO)4]4-
[Pt(NH3)4]2+
+ Na/liq-NH3  [Pt(NH3)4]
Reduction of salts of Group IV and V elements give
polyhedral anions, many examples.
Ge94-, Sn52-,
Sn93-, Pb52-, Bi42-,
P72- As64-
[Pt(NH3)4]
Reduction of salts of Group IV and V elements give
polyhedral anions, many examples.
Ge94-, Sn52-,
Sn93-, Pb52-, Bi42-,
P72- As64-