Nitrides
Many interstitial nitrides, chemistry not well developed.
Nitrogen atoms in holes in metal structures.
N  N3-
Requires lots of energy => need small cation
to stablise structure.
(Madelung Energy)
Hence, the only Group 1 or 2 nitrides are Li3N,
and Mg3N2
N3-
Requires lots of energy => need small cation
to stablise structure.
(Madelung Energy)
Hence, the only Group 1 or 2 nitrides are Li3N,
and Mg3N2
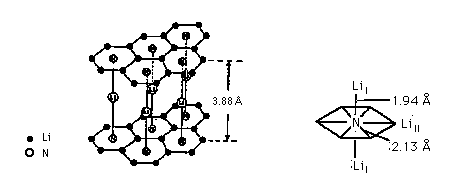 Figure 1:
The layer structure of Li3N, more correctly formulated as Li[Li2N],
has hexagonal Li6 nets.
The nitrogen has hexagonal bipyramidal coordination,
Li3N has high electrical conductivity.
Figure 1:
The layer structure of Li3N, more correctly formulated as Li[Li2N],
has hexagonal Li6 nets.
The nitrogen has hexagonal bipyramidal coordination,
Li3N has high electrical conductivity.
