Nitrogen Hydrides
NH3, N2H4,
N2H2
NH3 only thermally stable hydride.
Prepared industrially by the Haber process
(500 oC, high pressure),
naturally by nitrogen fixation.
Uses of Ammonia
4NH3 + 5O2  4NO + 4H2O (Huge scale)
NH3 + NaOCl
4NO + 4H2O (Huge scale)
NH3 + NaOCl  NH2Cl + NaOH
Chloramine
NH3 + NH2Cl
NH2Cl + NaOH
Chloramine
NH3 + NH2Cl  N2H4 + NaCl + H2O (Raschig process)
Side reaction
2NH2Cl + N2H4
N2H4 + NaCl + H2O (Raschig process)
Side reaction
2NH2Cl + N2H4  N2 + NH4Cl
catalysed by traces (ppm) of metal ions, add
gelatin.
Structure
N2 + NH4Cl
catalysed by traces (ppm) of metal ions, add
gelatin.
Structure
 Acid/Base properties
NH3 basic N2H4,
weakly basic
NH4+ N2H5+,
N2H62+
Redox properties
Both NH3 and N2H4
mild reducing agent (ox states -3 and -2).
Reactions complex in aq solution
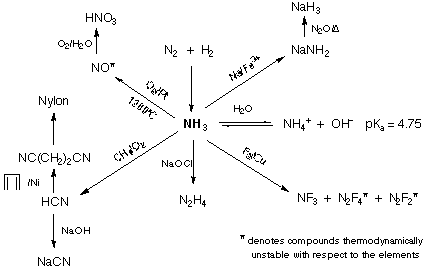
Summary of reactivity
Acid/Base properties
NH3 basic N2H4,
weakly basic
NH4+ N2H5+,
N2H62+
Redox properties
Both NH3 and N2H4
mild reducing agent (ox states -3 and -2).
Reactions complex in aq solution
Summary of reactivity