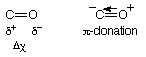
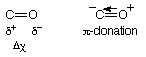
Overall small dipole moment, negative end on carbon atom.
- Molecular orbital diagram: 5s HOMO lone pair on carbon atom.
=> Lewis base(weak), need strong Lewis acid.
eg. ![]()
- Bonding to metal strengthened by p back donation.
- Synergic bonding.

- Need strong donor, which means need a metal in a low oxidation state.
Ni(CO)4, Fe(CO)5, Mn(CO)5Cl
- C-O bond weakened, easier to stretch, u(C ![]() O) lowerer compared to free CO.
O) lowerer compared to free CO.
- other p-acids, any potentially any species with p-bonds(!).
eg. N2, NO, O2