 CO2
entropically neutral =? flat.
CO2
entropically neutral =? flat.
Plot DGdeg of
oxide versus temperature => gradient = -DSdeg
- Linear (almost) except for kinks at phase changes
cf. Mg, Zn lines
Use for extraction of metals.
- If line is above those involving carbon, the metal oxide can be reduced.
- C + O2  CO2
entropically neutral =? flat.
CO2
entropically neutral =? flat.
- 2CO + O2  2CO2
2CO2
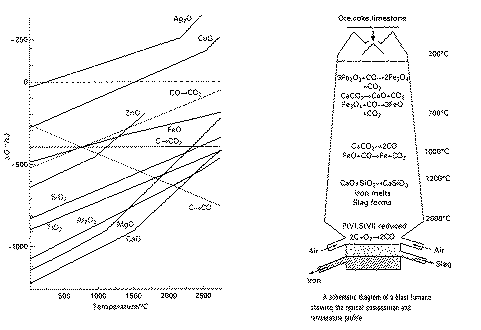
- At 983K: For FeO: below 983K only CO reduces FeO
above 983K, CO and C reduce FeO
=> easier to get coke than CO so therefore we work at 1000K
-Above 1400K, C + O2 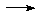 CO
can reduce Cr2O3
CO
can reduce Cr2O3
=> Cr2O3/FeO/C at 1400K so we get blue steel
- Zn, Fe, Pb made using C as reducing agent.
- Not MgO, or Al2O3
Copyright Dr. D. O'Hare 1996.