For the reaction:
E2H6  EH4 + E + H2
EH4 + E + H2
DH = B(E-E) + 2B(E-H) - DHatom(E)
- B(H-H)
Long chain C-C stable. due to strong C-C and C-H bonds.
For the reaction:
E2H6  EH4 + E + H2
EH4 + E + H2
DH = B(E-E) + 2B(E-H) - DHatom(E)
- B(H-H)
Long chain C-C stable. due to strong C-C and C-H bonds.
So could expect C=C but not Si=Si => no silicon analogues to graphite.
2B(E-E) > B(E=E) for both Si and C
=> C=C, Si=Si unstable with respect to E-E
=> Kinetic stability in C again: So ethene is unstable with respect to polyethene but needs a catalyst.
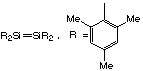
Compounds containing Si=Si double bonds are now known: They are stabilised
by using sterically bulky ligands eg. Also CO2 molecular,
CO32- isolated ions => Intramolecular p-bonding
SiO2, silicates are "polymerised => Internals-bonds
strong
B(C=O) < 2B(C-O) but B(Si=O) > 2B(Si-O)
Copyright Dr. D. O'Hare 1996.