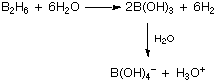Boron Hydrides
Many boron hydrides known, B2H6
only is important (at this level).
Two points on the synthesis. BF3/NaBH4
best method for producing B2H6.
Alfred Stock's original method only used for hexaborane-10.
MgB2 + H2O  B6H10
Properties
Stability; all endothermic (thermally unstable) compounds
=> kinetic stability.
Oxidation states < 3 => B-B bonding.
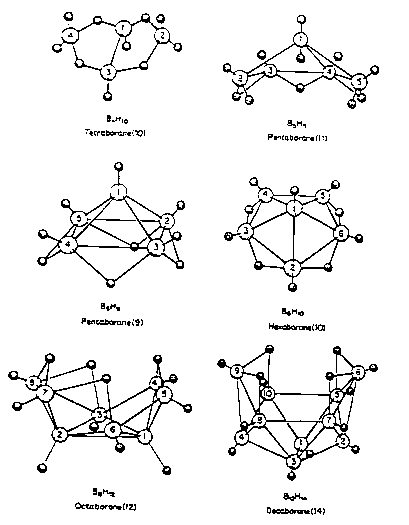
Structure and bonding is complex (Noble Prize).
Redox none on aqueous solution, but are reducing
agents.
B-H
B6H10
Properties
Stability; all endothermic (thermally unstable) compounds
=> kinetic stability.
Oxidation states < 3 => B-B bonding.
Structure and bonding is complex (Noble Prize).
Redox none on aqueous solution, but are reducing
agents.
B-H  B-O favourable; transfers
hydride ligand => reducing.
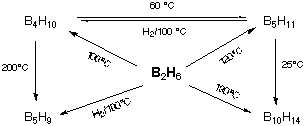
Pyrolysis of B2H6 under controled
conditions give higher boranes:
B-O favourable; transfers
hydride ligand => reducing.
Pyrolysis of B2H6 under controled
conditions give higher boranes:
Acid/Base Properties
All are acidic
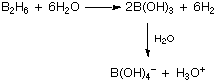 And many Lewis acid/base complexes known
And many Lewis acid/base complexes known
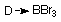 ; D = CO, PMe3, NMe3, Py etc...
; D = CO, PMe3, NMe3, Py etc...
 B6H10
B6H10 B6H10
B6H10 B-O favourable; transfers
hydride ligand => reducing.
B-O favourable; transfers
hydride ligand => reducing.