Boron Nitrogen Compounds
Three important compounds:
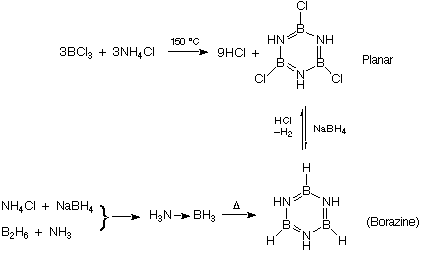 B=N isoelectronic with C=C => borazine is like benzene
(aromatic).
planar B3N3 ring, all B-N bonds
same lengths.
Due to polarity, much more reactive than benzene.
Borazine
B=N isoelectronic with C=C => borazine is like benzene
(aromatic).
planar B3N3 ring, all B-N bonds
same lengths.
Due to polarity, much more reactive than benzene.
Borazine  Boron nitride (BN)x
hexagonal
Boron nitride (BN)x
hexagonal  like graphite
structure (layered)
cubic
like graphite
structure (layered)
cubic  like diamond (hard)
like diamond (hard)
Hexagonal (BN)x
versus Graphite
Graphite: carbon in one layer on top of a ring.
(staggered or out of registery)
Hexagonal (BN)x: B above N interlayer
interaction.
(eclipsed or in registery)
weak B-N interlayer interaction.
(BN)x less easy to cleave.
polar B-N bond means that electrons are localised,
and so
(BN)x is an electrical insulator.


 Boron nitride (BN)x
Boron nitride (BN)x like graphite
structure (layered)
like graphite
structure (layered) like diamond (hard)
like diamond (hard)