|
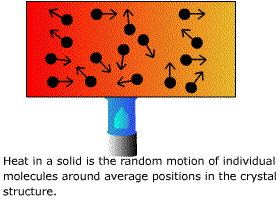
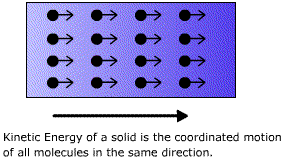
How do we measure disorder? The means of doing this came originally from physics, not chemistry. During the middle of the last century, physicists were interested in the nature of heat and its manipulation, an understandable bias in an era of steam power. James Joule, Julius Mayer, and others concluded after careful experimental measurements that heat, work, and energy all were merely different aspects of the same thing. William Thomson (later Lord Kelvin, of the Kelvin or absolute temperature scale) and Rudolf Clausius were struck by the fact that the interconversion of heat and work is a one-way street. It is easy to convert the energy of work completely into heat, but the reverse transformation is never complete. Thomson's version of the second law of thermodynamics states that it is impossible by any cyclic, repeatable process to take heat and convert it entirely into work without losing some of this heat to a reservoir at a lower temperature. There can be no steam engines without condensing cylinders, and part of the available heat always is lost to the condenser instead of being converted to useful work. The second law in any of its forms makes heat look like the lowest or most degraded form of energy: easy to obtain but hard to reconvert. We know what Thomson and Clausius a century ago did not. On a molecular level, kinetic energy is the coordinated motion of all of the molecules in a solid in the same direction (right). Heat in a solid is the disunited motion of individual moleculed about their equilibrium positions. Kinetic energy is organised, coherent motion and heat is random incoherent motion. |
|