| Decomposition
of N205
As a final example, let us examine the exception that showed the
flaws in the principle of energy minimization alone.
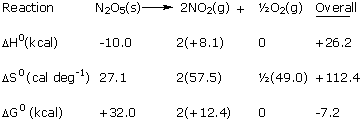 |
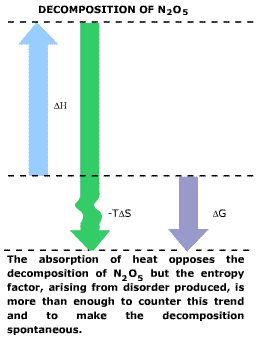
| The enormous entropy increase comes from the breaking up of
crystalline N205 into gases. Heat is absorbed, disfavoring the
reaction by 26.2 kcal. The entropy increase favors reaction
by the amount. |
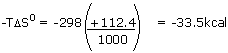 |
|
|
