|
When working with enthalpies, entropies, and free energies, it
is convenient to write the reaction equation first and then tabulate
the individual values below each component of the reaction.
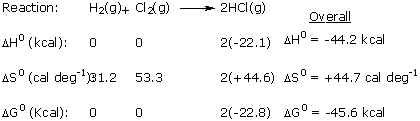 |
|
Note that the standard free energies of formation of elements,
like their standard heats of formation, are zero by definition
because the elements already are in their standard states.
The entropies, in contrast, are not entropies of formation
from elements, but are absolute measures of disorder. Hence
the elements have molar entropies just as the molecular compounds
do. As a check, we can verify that
|
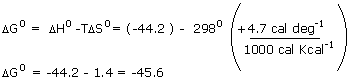 |
|
|
