|
In
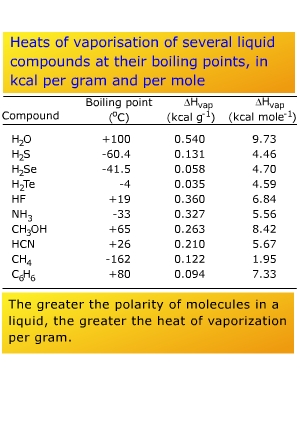
the series O-S-Se-Te down Group VIA of the periodic table, hydrogen
bonding becomes unimportant, because the atoms of S, Se, and Te
are too large and their negative charge is too diffuse to attract
a proton of a neighboring molecule strongly. Hence H2S,
H2Se, and H2Te
have more "normal" boiling points and heats of vaporization
(see right, and next page).
This anomalously high heat of vaporization of water has major consequences
for life on this planet. Evaporation of ocean water in the tropics
keeps the equatorial regions from being as hot as they would be
otherwise, and the heat removed warms the more polar regions when
the water vapor condenses.
Liquid water therefore acts as a heat reservoir, moderating the
extremes of temperature both at the equator and near the poles.
The evaporation of ocean water at the equator appears to be approximately
2.3 meters of depth per year, corresponding to a removal of 1.3
trillion (1,300,000,000,000) kilocalories of heat per square kilometer
of surface area!
|
|
