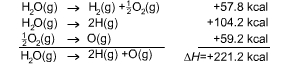|
We must correct for the energy absorbed in taking these diatomic
molecules apart: |
This is what we were after: the energy required to tear a
water molecule apart, not into elements in their standard
states, but into isolated atoms. Half of this total energy
can then be ascribed to each bond, giving an O-H bond energy
of 110.6 kcal per mole of bonds. |
|
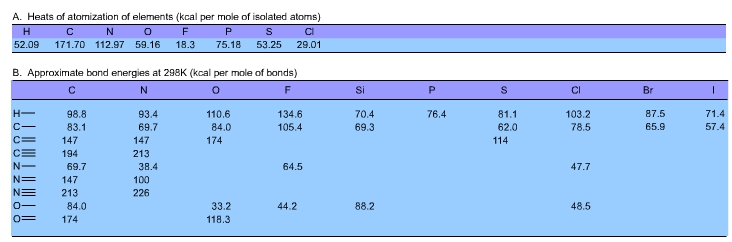 |
||