|
For
a general reaction of the type A + 4B �
3C + 2D, in which A, B, C, and D are chemical substances, the heat
of reaction can be calculated from DH0
= 3DH0C
+ 2DH0D
- DH0A
- 4DH0B,
in which DH0C
is the heat of formation of Compound C from its elements in their
standard states.
For example, if the reaction is the oxidation of glucose by nitrate,
as is carried out by some nitrate-respiring bacteria
5C6H12O6+24KNO3
� 6CO2+24KHCO3+12N2+18H2O
then the heat of reaction is obtained from the following combination
of heats of formation of products and reactants:
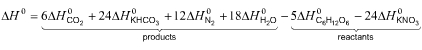
Tables of standard heats of formation can be found in the CRC Handbook
of Chemistry and Physics, in Lange's Handbook of Chemistry. With such
tables, the heats of all reactions involving the compounds can be
calculated, including reactions that never have been carried out,
or that for various reasons we cannot carry out easily in the laboratory.
|
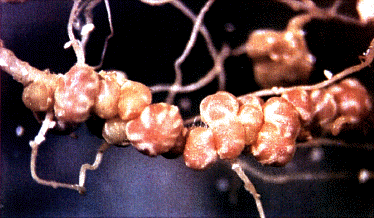
Nitrogen fixing nodules on a bean root
formed by the Rhizobium etli bacterium
|