Heats of reactions are additive in exactly the same way that the
reactions they belong to are additive. From the information in the
heat of combustion table, we can calculate how much heat would be
given off by the combustion of a mole of water in fluorine gas,
even though this reaction is not found in the table:
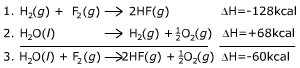 |
This calculation tells us that 60 calories more heat are obtained
by burning hydrogen in F2
(Reaction 1) than in O2
(reverse of Reaction 2). Even the "garbage" from O2
combustion (H2O) still
is a fuel for F2 combustion
(Reaction 3), because F is more electronegative than O.
This additivity of heats of reaction is a tremendous labour-saving
device. One might think that, to have available complete heat of
reaction data for all possible chemical reactions, it would be necessary
to measure and tabulate all of these heats. This is not the case.
It is necessary to tabulate heats only for the minimum number of
reactions from which all other reactions can be obtained by suitable
combinations.