|

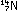 
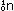 
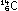 

    
    
To a first approximation, the carbon isotope ratio in the atmosphere
and in all living organisms is fixed.
As long as the organism is alive, it maintains this same carbon-14
to carbon-12 ratio. But as soon as it dies, exchange with the atmosphere
ceases. The slow decay of carbon-14 causes the isotope ratio to
diminish by half every 5570 years. It is a simple matter in principle,
although careful work is required to prevent contamination, to determine
the actual carbon isotope ratio in any ancient sample of wood or
other preserved organic matter, and to calculate from this how long
ago the specimen died. In this way, surprisingly accurate chronologies
can be constructed for the past 10,000 years. Radiocarbon dating
has become one of the most important tools of archaeologists and
palaentologists.
Similar dating methods can be used for geologic time spans by picking
isotopes with longer half-lives. Potassium-40 decays with a half-life
of 1.3 billion years to argon-40. Uranium-238 decays to lead-206
in a series of steps, of which the slowest has a half-life of 4.5
billion years, and rubidium-87 decays with a half-life of 47.5 billion
years to strontium-87. Dating rock samples from the Earth and moon,
and meteorite fragments, via potassium-argon, uranium-lead, and
rubidium-strontium methods, has been an important tool in the study
of our solar system.
|


|

