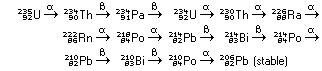|
A fourth kind of decay is
observed in heavy nuclei that have too many of both protons
and neutrons. Such nuclei are found at the upper right end
of the stability plot. These nuclei decay by giving off an
alpha particle, or helium nucleus ( |
by four, and the atomic number
decreases by two. The product nucleus also may be unstable, and
if so, a second and a third decay step can occur. Uranium-235 begins
a multistep decay chain, which eventually stops at a stable isotope
of lead: |
|