|
The
iron half-reaction is simple:
 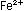
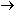 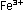
 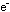
    
    
The final step is to add these two individually balanced half-reactions
and obtain an overall reaction in which electrons do not appear
explicitly. To accomplish this, we must add one unit of the manganese
reaction to five units of the iron reaction. The result is the same
as before:
 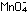
 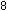 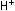
 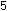 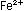
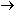 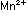
 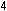 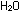
 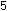 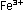
    
This overall equation is now balanced with respect to charge and
number of atoms because the half-reactions were balanced, and with
respect to oxidation number because the proper multiples of the
half-reactions were chosen to make the electrons cancel.
|
|