|
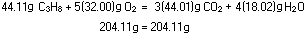
The balanced equation indicates that each mole of propane gas burned
requires five moles of oxygen. The problem as stated involved 1000g
of propane, and the number of moles is
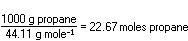
Five times this many moles of oxygen are needed:
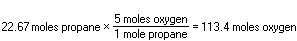
The quantity of oxygen in grams then is
113.4 moles O2 x 32.00
g mole-1 = 3629 g O2
The entire calculation could have been set up in one step:

|
A single
molecule of propane
Formula C3H8
|