|
You
should verify the relationship by checking one of the previous examples.
In the HCl reaction 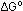 (298) = -45.54 kcal per 2 moles of HCl. Then,
(298) = -45.54 kcal per 2 moles of HCl. Then,
 = 10
= 10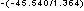 = 2.5 x 10
= 2.5 x 10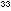
This tallies with the value of  quoted previously, which is necessarily so since this is how the
value was obtained originally. For many reactions with extremely
large or small equilibrium constants, it is easier and more accurate
to measure the free energy change of the reaction and use the equations
given above, than to try to obtain
quoted previously, which is necessarily so since this is how the
value was obtained originally. For many reactions with extremely
large or small equilibrium constants, it is easier and more accurate
to measure the free energy change of the reaction and use the equations
given above, than to try to obtain  from concentration measurements at equilibrium.
from concentration measurements at equilibrium.
At temperatures other than 298K, the 1.364 factor cannot be used.
For the ammonia reaction at 1000K:
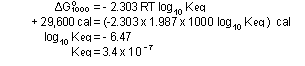
This was the value of  quoted previously. Note that
quoted previously. Note that 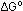 (1000) was expressed in calories instead of kilocalories because
R is in calories per degree per mole.
(1000) was expressed in calories instead of kilocalories because
R is in calories per degree per mole.
|
|