|
The synthesis of ammonia gives off heat:
 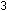
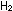 (g) +
(g) + 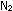 (g)
(g) 
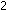
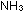
 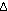 H H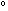 (298) = -22.1 kcal per 2 moles of
(298) = -22.1 kcal per 2 moles of 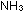
Ammonia molecules absorb heat when they dissociate.
The energy released in forming one N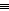 N
and three N
and three
H H
bonds is not enough to break apart six N H
bonds is not enough to break apart six N H
bonds in the two ammonia molecules. H
bonds in the two ammonia molecules.
Raising the temperature therefore should favor dissociation, because
the extra thermal energy is put to work pulling molecules apart.
This is borne out by the measured values of 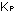 for the reaction.
for the reaction.
|
|