|
Equilibrium-constant
calculations involving gases quickly become more complicated than
the ideas they were intended to illustrate, and we will defer most
equilibrium calculations to the discussion of aqueous solutions
in Chapter 16. However, it is useful to look briefly at the equilibrium
constants for some of the reactions discussed previously in this
chapter. Along the way we will encounter some of the fundamental
ideas about manipulating equilibrium-constant expressions.
HCl Synthesis
The equilibrium-constant expression for the HCl reaction has the
same form as that of HI synthesis:
 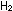 (g) +
(g) + 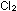 (g)
(g) 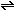 2HCl
(g) 2HCl
(g)
 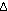 G G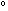 = -45.54 kcal per 2 moles of HCl
= -45.54 kcal per 2 moles of HCl
The experimental value of K, for this reaction is 2.5 X 10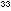 . Notice that, with equal power of concentration terms in both numerator
and denominator,
. Notice that, with equal power of concentration terms in both numerator
and denominator,  for HCl is a unitless quantity.
for HCl is a unitless quantity.
|
|
 for this
reaction will have the same numerical value whether concentrations
are measured in moles per liter, mole fractions, partial pressures,
or any other convenient system ( for this
reaction will have the same numerical value whether concentrations
are measured in moles per liter, mole fractions, partial pressures,
or any other convenient system ( =
= 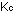 = = 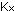 =
= 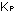 ). This is
not always true. ). This is
not always true.
If we begin with equal concentrations of 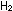 and
and 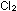 ,
then since they react in equal amounts, the concentrations of these
two substances always will be equal, [ ,
then since they react in equal amounts, the concentrations of these
two substances always will be equal, [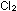 ]
= [ ]
= [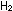 ],
and at equilibrium we can write: ],
and at equilibrium we can write:
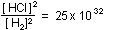 or or
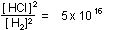
This tells us that equilibrium will not be reached until the ratio
of HCl to 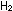 ,
(or ,
(or 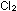 )
has risen to 50 million billion to one! It is not surprising that
we cannot detect any )
has risen to 50 million billion to one! It is not surprising that
we cannot detect any 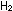 or
or 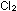 in
the products at equilibrium. in
the products at equilibrium.
So large an equilibrium constant indicates that the reaction starting
with equal concentrations of 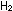 , ,
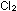 , and
HCl should be highly spontaneous, since the reaction has a long
way to go before reaching equilibrium. The large negative standard
free energy change, , and
HCl should be highly spontaneous, since the reaction has a long
way to go before reaching equilibrium. The large negative standard
free energy change, 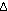 G G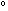 =
-45.54 kcal per two moles of HCl, indicates the same thing. =
-45.54 kcal per two moles of HCl, indicates the same thing.
|