|
If the interaction were a simple
collision of two molecules (we will discuss the actual mechanism
in the next chapter), then doubling the concentration of either
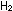 or or 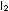 would double the rate of reaction, and doubling both at the same
time would make the collisions, and hence the reaction, occur four
times as fast. The rate of forward reaction then would be given
by
would double the rate of reaction, and doubling both at the same
time would make the collisions, and hence the reaction, occur four
times as fast. The rate of forward reaction then would be given
by
  =
= 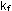 [ [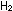 ]
[ ]
[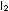 ] ]
|
|
This expression says that the rate
of forward reaction is proportional to each of the reactant concentrations
independently, with a proportionality constant, 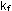 .
If the rate is measured in moles per second, and concentrations
are in moles per liter, then .
If the rate is measured in moles per second, and concentrations
are in moles per liter, then 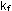 has units of liter
has units of liter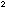
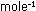 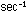 . These rather strange units for
. These rather strange units for 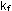 mean that as one type of molecule sweeps through the available volume,
colliding with other molecules, its rate of sweep can be expressed
in liters per second, per unit of concentration (moles per liter)
for that type of molecule.
mean that as one type of molecule sweeps through the available volume,
colliding with other molecules, its rate of sweep can be expressed
in liters per second, per unit of concentration (moles per liter)
for that type of molecule.
|