Sodium Through Argon
|
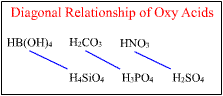
Nitrous acid, HNO2, is weaker than nitric acid, HNO3; and phosphorous acid, H3PO3, is weaker than phosphoric acid, H3PO4. Sulfurous acid, H2SO3, is not as strong as H2SO4. Chlorine can form an entire series of oxyacids with decreasing oxygen content and decreasing acid strength:
In each of these acids, shown at the left, the dissociating
proton is bonded to an oxygen atom; thus the second structure given
above for each acid is a more accurate model of the molecule. |
The greater the number of oxygen atoms bonded to the Cl, the greater is the attraction by the rest of the molecule for the electrons of the W-O bond, and the more likely the acid molecule is to dissociate into H+ and an oxyanion. Perchloric acid is the strongest common inorganic acid known, but bypochlorous acid is weak. The third-row nonmetals also form acids other than oxyacids, in which the protons are attached directly to the central atom, as in HF. Hydrochloric acid, HCl, is a strong acid and, with HNO3 and H2SO4, makes up the trio of acids that you are most likely to encounter in the laboratory. Sulfur is less electronegative than chlorine, therefore hydrogen sulfide, H2S, is a much weaker acid. Even so, it is stronger than its second-row analogue, H2O. This is so because the sulfur atom is large enough that the protons
cannot get as close to the center of charge on the sulfur atom,
and the attraction between S and H is weakened. |