Sodium Through Argon
|
In comparing carbon in the second row with silicon in the third, we were looking at a common theme with striking variations. These arose from the larger size of Si and the lesser ability to form multiple bonds with like neighbor atoms. This trend also is seen in comparing nitrogen (N 2,5) with phosphorus (P 2,8,5), oxygen (O 2,6) with sulfur (S 2,8,6), and fluorine (F 2,7) with chlorine (Cl 2,8,7).
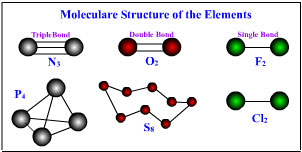
Nitrogen gas, N2, has a triple bond between two atoms, with a lone electron pair on each atom. Phosphorus atoms are too large to form triple bonds, so in P4 molecules, |
which are found in the gas, liquid, and one solid form of phosphorus, each P atom makes a single bond to three others at the corners of a tetrahedron (below). Similarly, oxygen gas has a double bond in the O, molecule, but solid sulfur is made up of eight-membered rings Of S8, in which each S atom is singly bonded to two others in the ring. Chlorine is singly bonded in Cl2 gas molecules, as is F2. As we saw in the preceding chapter, carbonic acid is a weak acid. Carbon has room for no more than three oxygen atoms around it, as in the carbonate ion, CO32-.
|