Sodium Through Argon
|
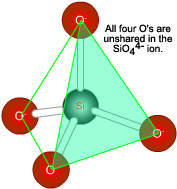
In the center of the third row of the periodic table sits silicon, with four outer electrons like carbon. The rocks of our planet are derived from silicon dioxide, SiO2, and are surrounded by an atmosphere composed in part of carbon dioxide gas, CO2. This does not seem remarkable until we recognize that silicon and carbon, which have the same outer electronic structure, should have similar chemical properties. Why the gross difference in properties of their oxides? The difference in properties arises because a silicon atom is larger than a carbon atom. Sharing two electron pairs with another atom in a double bond requires a closer approach of atoms than for a single bond. Silicon, with an inner core of ten electrons, cannot get close enough. Carbon, with a two-electron inner core, is smaller and can make C=O bonds. Two such double bonds build a CO2 molecule, O=C=0. Rather than making double bonds to two oxygen atoms, it is easier for silicon to make single bonds to four oxygens, arranged around the Si atom at the corners of a tetrahedron. |
Each of these oxygen atoms can bridge two silicon atoms, and the result is an endless threedimensional lattice of silicate tetrahedra as in quartz, shown on the next page. If silicon were smaller and could make double bonds to oxygen, there would be no reason not to expect discrete molecules of O=Si=0. Quartz would be a gas instead of a very hard mineral, and the history of our planet would be vastly different. There is another factor: the smaller electronegativity of Si as compared with 0, 1.8 versus 3.5. |