Sodium Through Argon
|
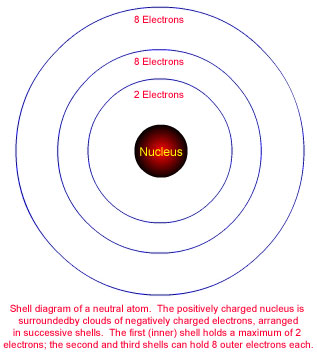
The electronic structures of the first eighteen elements are shown on the opening page, arranged in three rows of the periodic table as it was introduced at the end of Chapter 3. These are not pictures of the atoms, but are schematic diagrams of electron-shell structure. Electrons are represented by black dots. Each shell is shown in white while it is being filled, and with a colored background when full, so that attention will be focused on the next shell being filled. The second and third shells are divided into four boxes as a reminder that the eight electrons in a full shell occur as four electron pairs. Below each shell diagram is a Lewis electron-dot diagram of the atom, which conveys the same information about outer-shell electrons. Each of the four compass points around the atom symbol in a Lewis diagram represents one of the four possible positions for electron pairs. |
|