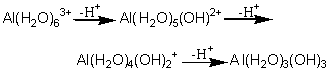Sodium Through Argon
|
The attractions between hydrating water molecules and the central ion are mainly electrostatic for Na+ and Mg2+, as they are for Li+. For Al3+, as for Be2+ in the second row, the metal-oxygen bonds have a more covalent character, which in turn weakens the O-H bonds of the hydrating water molecules. Electrostatic repulsion between ions plays a large part in keeping AI(H2O)63+ or AI(OH )63- ions in solution. If we begin with an acid solution of hydrated Al3+ ions and gradually lower the acidity, or amount of 11+ present, several of the weakened water molecules coordinated to aluminum will give off a proton each, leaving hydroxide ions bound to the metal. The charge on the complex ion will decrease by one each time a proton is released:
|
With no electrostatic repulsion to keep the ions apart in solution, aluminum hydroxide comes out of solution as a gelatinous, watercontaining precipitate. The same behavior is seen if a basic solution of AI(OH)63- ions is gradually acidified. Some of the added protons combine with hydroxide ions and turn them into water molecules. The charge on the ions is gradually reduced, and hydrated Al(OH)3 again precipitates as a gel. Similar behavior was mentioned for beryllium in Chapter 5. Be(H2O)42+ ions are soluble in acid, and Be(OH)42- in base; but beryllium oxide s insoluble in pure water. Only four groups are coordinated around beryllium, rather than six, because Be2+ is smaller than Al3+. Both beryllium and aluminum oxides are amphoteric, and are at the
borderline between basic and acidic oxides in their respective rows. |