|
With the formation of succinate, the two big energy-releasing and C02-producing steps of the cycle are over, and the original six-carbon citrate has been degraded to a four-carbon molecule. However, more energy is still available. Succinate is oxidized to fumarate with the storage of energy in FADH2, fumarate is rearranged to malate, and malate finally is oxidized to oxaloacetate with the simultaneous reduction of NAD+. The cycle is completed when oxaloacetate combines with acetyl CoA and another turn of the wheel begins. The unfinished business is the machinery for reoxidizing NADH and FADH2 and making use of their energy. This is the topic of the next section. At this point we can stop and draw a balance sheet of the entire energy situation, from glycolysis through the citric acid cycle.
|
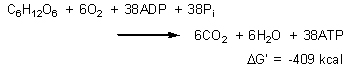
This is equivalent to a total of (4 X 1) + (10 X 3) + (2 X 2) = 38 ATP molecules per molecule of glucose. Of the total 686 kcal released per mole of glucose, 38 X 7.3 = 277 kcal are saved, a 40% overall efficiency. The other 409 kcal are not entirely useless. They ensure the thermodynamic spontaneity of the reaction, and provide body heat:
Several energy-producing pathways besides glycolysis funnel together and enter the citric acid cycle to produce energy. When fats are used as an energy source, the fatty acids are broken down into two-carbon acetate and fed into the cycle. During the metabolism of proteins, some amino acids are converted into pyruvate or acetate and then enter the cycle. Thus the biochemical machinery that probably evolved to make maximum use of the products of glycolysis now is used with many other processes. Any molecule that can be broken down to acetate can enter the citric acid cycle and yield energy. |