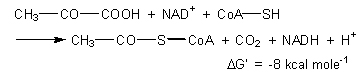|
The compound that enters the cycle, acetyl coenzyme A, is 7.5 kcal higher in energy than simple acetic acid is, and hence is better able to start the cycle:
One precycle step is necessary to turn pyruvate into acetyl coenzyme A (Step 1 on next page). This is an oxidation step in which three things happen at once: pyruvate is oxidized to acetate with the release of C02, some of the energy from oxidation is saved by reducing NAD+ to NADH, and part of the leftover energy is stored temporarily by adding coenzyme A (CoA) to the acetate. The same three-for-one reaction occurred in glycolysis when G3P was converted to DPG. |
In that process an aldehyde was oxidized to an ester, some of the energy released by oxidation was stored in NADH, and some of the remaining energy was preserved in a second phosphate bond in the molecule. A good metabolic idea is too valuable not to use more than once. We shall see it a third time in the citric acid cycle. The energy stored temporarily in acetyl coenzyme A is used to get the citric acid cycle started by a reaction with oxaloacetate to make citrate. When this happens, the coenzyme molecule falls away, to be recycled and bound to another acetate. The overall oxidation of pyruvate to acetate releases 68 kcal mole-1 of free energy. Of this energy, 52.5 kcal are saved in the NADH formed, 7.5 kcal are stored in the acetyl CoA complex, and 8 kcal are left over to ensure that the reaction remains spontaneous and does not back up:
|
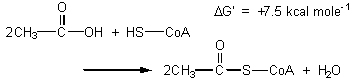 The
logic behind this priming step is the same as that for priming glucose
to G3P in the early steps of glycolysis. The structure of coenzyme
A is shown on the
The
logic behind this priming step is the same as that for priming glucose
to G3P in the early steps of glycolysis. The structure of coenzyme
A is shown on the