|
For every mole of glucose that we burn we obtain 673 kcal of heat: The products are more disordered than the reactants, so we get
an extra 13 kcal mole-1 of free energy "push"
from the increase in entropy: The total free energy of the reaction is
This free energy is the potential driving force for other chemical reactions. If combustion were a one-step process, it would be hopelessly wasteful. There is no efficient way to lock up 686 kcal of chemical energy at one time, in a way that will be useful later. The free energy must be stored in smaller pieces. This is the reason that glucose is processed through a complex set of biochemical reactions instead of merely touching a match to it. Part of the process is to break glucose down in a series of small steps, thereby releasing less free energy at any one step. |
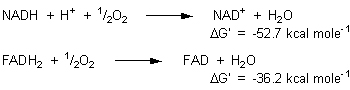
Another trick is to carry out the coupling in two stages - to remove free energy in larger units than the 7.3 kcal mole-1 associated with ATP, and to use these units to make several ATP molecules in a separate series of reactions. NAD+ and FAD, which were discussed in Chapter 22, are the means of removal of these larger blocks of energy. If oxygen is the oxidizing agent, then one mole of NADH can be thought of as carrying 52.7 kcal of free energy, and one mole of FADH2, 36.2 kcal. These are the amounts of free energy that are released when the reduced carriers are reoxidized:
Every reoxidation of NADH leads to the formation of 3 moles of ATP, with the storage of 3 X 7.3 kcal = 21.9 kcal of free energy. Saving 21.9 kcal out of a total of 52.7 kcal represents a 42% efficiency of energy conversion, which is reasonably typical for biological processes. The reoxidation of FADH2 leads to the synthesis of two ATP molecules and the saving of 2 X 7.3 = 14.6 kcal of energy, which is a 40% energy conversion. |