|
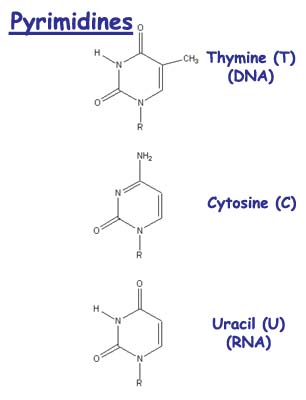
As was first mentioned in Chapter 10, the free energy of hydrolysis of ATP into ADP and inorganic phosphate is unusually high for organic phosphate compounds, around 7.3 kcal mole-'. This is the energy that must be supplied to produce ATP and water from ADP and phosphate, and this is the free energy that is released again when ATP is hydrolyzed. The further hydrolysis of ADP to AMP and phosphate releases a similar amount of energy, but the free energy of hydrolysis of AMP to adenosine and phosphate is only 3.4 kcal mole-', which is similar to that of other organic phosphate compounds. The unusually large hydrolysis energies, which arise partly from delocalization of electrons and partly from repulsions between negative charges on the polyphosphate groups, make ATP a useful means of storing chemical energy in living systems. No matter how a particular organism obtains its chemical energy, or what compounds it employs for long-term energy storage, every living organism first converts chemical energy into ATP molecules, and then uses this ATP for its subsequent purposes. It is tempting to think that life began as a scavenger of ATP from the primordial seas, and that all the other energy-gathering processes developed only as alternative ways of making artificial ATP when the natural supply ran out. Right: The two pyrimidines in DNA, cytosine and thymine, have single rings. In RNA, thymine is replaced by uracil, which does not have the thymine methyl group. |
|