|
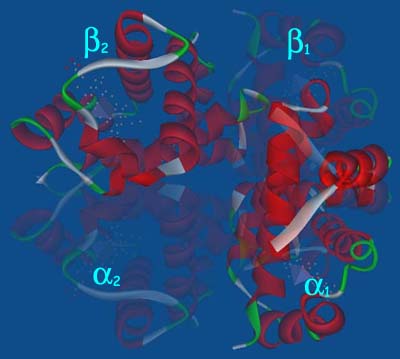
The hemoglobin molecule(right) is essentially four myoglobin molecules put together. Each hemoglobin molecule has four separate protein chains: two a chains and two b chains. Each of these chains is folded in the same way as the myoglobin molecule, and the four chains are nested against one another as four subunits of a compact molecule. When the hemoglobin molecule picks up four 02 molecules at the lungs, the subunits shift slightly so the two b units are a little closer together. When the 02 Molecules are turned over to myoglobin at the tissues for storage, the four hemoglobin subunits shift back to their original arrangement. In effect, the hemoglobin molecule is a machine that closes and opens when it binds and releases oxygen. Interactions between subunits also make binding of oxygen to hemoglobin an all-or-nothing proposition. Once an 02 molecule has bound to one of the four heme groups, a subunit shift makes it much easier to add the other three 02 molecules. Conversely, once one 02 molecule has been released at the tissues, the other three fall away more easily.' This makes the hemoglobin molecule easy to load with 02 at the lungs and easy to strip of its cargo at the tissue, properties desirable in an oxygen carrier. |
|