|
The way in which the protein chain is folded in the myoglobin molecule
is shown (right). Myoglobin has 153 amino acids in one continuous
chain, and a molecular weight of 17,000. It is a relatively small
protein. For simplicity, only the alpha carbons of the main chain
are shown, and the -CO-NH- amide groups connecting them are represented
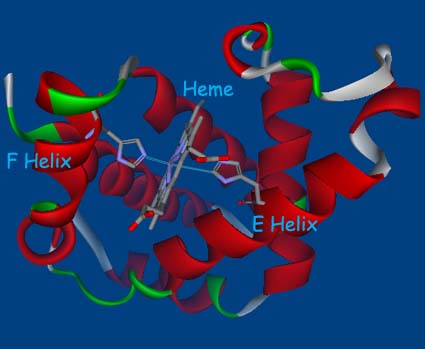
by a straight line. The chain is coiled into eight segments of cylindrical
a helix, identified by the letters A through H. A more schematic
diagram of the myoglobin molecule is shown above. The corners or
bends between helices are given the two letters of the helices that
they connect-corner AB between helices A and B, and so on. Only
by such abrupt elbow bends can an essentially linear fibrous structure
- the a helix - be fitted into a globular
protein of finite dimensions. The a helix occurs in myoglobin and
many other globular proteins because it is an efficient way to fold
a protein chain, but the price that must be paid is irregular bends
every so often along the chain. |
|