|
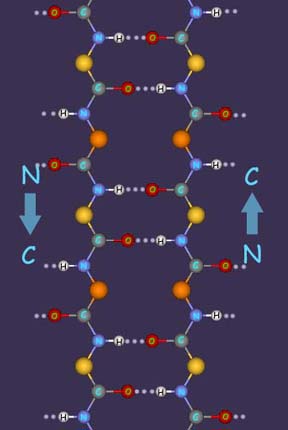
The slight negative charge on the carbonyl (-CO-) O atoms, and the slight positive charge on the amide (-NH-) H, are important in determining how a protein chain will fold, since a hydrogen bond can form between the 0 and the H in the way described for water molecules in Chapter 4. If two protein chains run parallel to one another in opposite directions, a large number of hydrogen bonds can be formed between them like rungs in a ladder (left). This is the way that protein chains are held together in silk, a fibrous protein. Many protein chains are packed next to one another in a sheet, with neighboring chains running in opposite directions and held together by hydrogen bonds. These sheets then are stacked together to build up a three-dimensional structure. Three different kinds of chemical forces are present in the three-dimensional silk structure. Along the protein chains (which also is the direction of the silk fibers) atoms are linked by covalent bonds. At right angles to these chains, within one sheet, the chains are held together by hydrogen bonds. These are weaker than covalent bonds (~6 kcal mole-1 compared to 80-100 kcal. mole-1) , but are important because there are so many of them. In the third dimension, the stacked sheets are held together by weak van der Waals forces between side chains, most of which are glycine and alanine in silk. Right: In silk and other insect fibers, antiparallel extended chains of protein are cross-linked into sheets by hydrogen bonds. These sheets then are packed into a three-dimensional structure. |
|