|
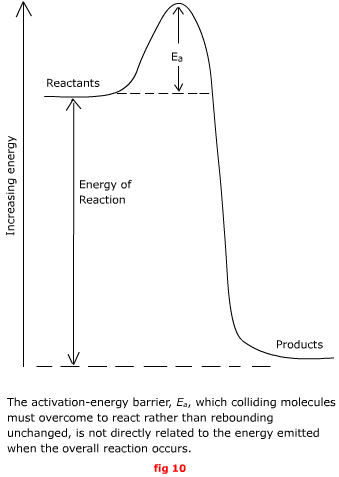
This threshold energy, Ea is called the energy of activation. It can be thought of as a barrier that the reaction must surmount before it can go to completion (see fig 10 opposite). From the usual random distribution of
energy among molecules, the fraction of molecules that have enough
kinetic energy to collide with energy Ea
or greater is This fraction increases with temperature.
At absolute zero, it has the value: As the temperature approaches infinity,
the fraction of molecules capable of reacting approaches unity,
no matter how large the activation energy, Ea,
might be: At any finite temperature, the larger
the activation energy, the smaller the fraction of molecules that
have enough energy to surmount this barrier and react. The simple
collision theory of chemical
reaction states that the rate
constant can be represented by
|
|