|
8. Of the three compounds with -OH groups, NaOH, 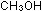 ,
and ,
and 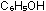 , one
is an acid, one is a base and one is neither. Explain in terms of
molecular structure and bonding. , one
is an acid, one is a base and one is neither. Explain in terms of
molecular structure and bonding.
9. What are structural isomers? Why do the isomers of 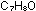 ,
which were mentioned in this chapter, have such different chemical
and physical properties? ,
which were mentioned in this chapter, have such different chemical
and physical properties?
10. What is an ether, and how is it made from alcohols? Why
is the ether that is made from polar alcohol molecules nonpolar?
How does this affect the solvent properties of ethers?
11. How are aldehydes made from alcohols? How are ketones
made?
12. Why are aldehydes and ketones polar, whereas ethers are
not? Why are aldehydes and ketones susceptible to chemical attack?
13. What compounds are produced when aldehydes are oxidized?
Can ketones be oxidized under similar comditions?
14. Why is CH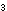 -CO-OH
an acid, whereas CH -CO-OH
an acid, whereas CH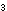 -CH -CH -OH
is not? How does the -CO- group contribute to making the molecule
acidic? -OH
is not? How does the -CO- group contribute to making the molecule
acidic?
|
|