|
6.
Give the systematic names of the following substances:
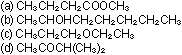
7. What is the Br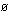 nsted-Lowry
conjugate base of phenol? Is aniline a B-L acid or base, and what
is its conjugate base or acid? nsted-Lowry
conjugate base of phenol? Is aniline a B-L acid or base, and what
is its conjugate base or acid?
8. What is the conjugate base of propionic acid? The 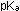 of propionic acid is 4.87. (a) Calculate the pH of a 0.1M
solution of propionic acid. (b) Calculate the pH of a solution
of 0.1M in propionic acid and 0.1M sodium propionate. (Refer to
Chapter 16 if necessary.)
of propionic acid is 4.87. (a) Calculate the pH of a 0.1M
solution of propionic acid. (b) Calculate the pH of a solution
of 0.1M in propionic acid and 0.1M sodium propionate. (Refer to
Chapter 16 if necessary.)
9. Write balanced chemical equations for the following: (a)
the hydrolysis of isopropyl acetate; (b) the reaction of
acetic acid and 2-butanol in the presence of sulphuric acid; and
(c) the reaction of methyl chloride with ammonia (more than
one product will be formed).
10. Arrange the following substances in order of increasing
acidity:
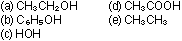
|
|