|
The alcohol functional
group, -OH, looks misleadingly like the hydroxide group of a base,
which might suggest that it dissociates from the rest of the molecule.
It cannot be emphasised too strongly that this is not so; an alcohol
is held firmly together by covalent bonds and does not dissociate.
This is because the oxygen atom and the carbon to which it is bonded
in the hydrocarbon radical are of approximately the same electronegativity.
If the carbon were much less electronegative than the oxygen, then
the oxygen of the -OH would pull the bonding electrons to itself
and the molecule would ionize as a base does:
 H H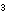 C C O O H H
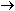 H H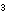 C C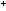 +
+ 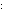 O O H H
This happens with NaOH, but not with CH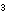 OH. OH.
|
|
In contrast, if the carbon
were much more elctronegative, then the alcohol would pull electrons
away from the O H
bond and release a proton, as an acid does: H
bond and release a proton, as an acid does:
 H H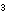 C C O O H H
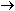 H H C C O O  + H
+ H
This occurs with O N N O O H
(nitric acid, HNO H
(nitric acid, HNO ),
but not with ),
but not with
H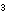 C C O O H
(methanol). H
(methanol).
|