|
n-Butyl
alcohol becomes 1-butanol and sec-butyl alcohol is 2-butanol,
but for isobutyl and tert-butyl alcohols a shorter carbon
skeleton than four atoms must be used. Isobutyl alcohol has the
systematic name of 2-methyl-1-propanol, or 2-methyl propanol, and
tert-butyl alcohol is 1,1-dimethyl ethanol.
Common names usually are employed for these small molecules because
they are simpler to write. But with more than four or five carbon
atoms, the systematic name becomes easier to remember, or to work
out from the molecular structure.
Alcohols are classified as primary, secondary, or tertiary, depending
on whether the carbon bearing the  OH
group is connected to one, two, or three other carbons. OH
group is connected to one, two, or three other carbons.
Of the alcohols illustrated on the previous page, all are primary
except isopropyl alcohol and sec-butyl alcohol, which are
secondary, and tert-butyl alcohol, which is tertiary.
|
|
Alcohols can be prepared
synthetically by the hydrolysis of chlorides and bromides of hydrocarbons:
 R R Cl
+ Cl
+ 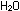 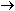 R
R OH + HCl OH + HCl
Since this reaction liberates HCl, it proceeds best in basic solution
where the acid can be neutralised as fast as it is made.
Many of the lower-molecular-weight alcohols are produced by yeasts,
bacteria, and other microorganisms as by-products in their energy-extracting
metabolism. Yeasts burn sugars to 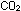 and water (as we do) if given enough oxygen, but under anaerobic
conditions they stop halfway and give off ethyl alcohol. as a waste
product.
and water (as we do) if given enough oxygen, but under anaerobic
conditions they stop halfway and give off ethyl alcohol. as a waste
product.
The ancient art of brewing has given ethyl alcohol (ethanol) the
name of "grain alcohol" because of its easiest and cheapest
source.
|