|
The composition of the universe reflects this evolutionary origin,
as shown in the table on page 32. The universe
is 93% hydrogen and 7% helium, with all of the other elements amounting
to only 0.10%. A gap in natural abundance exists between He and
C, reflecting the jump in synthesis:
 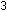
 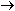
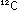                
Lithium, beryllium, and boron are formed later in secondary reactions
involving the breakup of larger nuclei. Hence they are scarce in
comparison with the elements on the direct line of synthesis. Beyond
carbon, there is an alternation of abundance, with atoms of even
atomic numbers more common than odd, as seen on the graph on page
31. Again, this reflects the synthesis of the even elements
by addition of alpha particles,  .
The odd elements, just like Li, Be, and B, must be made by side
reactions and hence are not as prevalent. This is the primary reason
why oxygen is used for the energy-producing reactions of life on
Earth, even though fluorine is more electronegative; fluorine is
simply too rare to be depended on as a biological oxidant. If this
were not so, we probably would burn our foods with .
The odd elements, just like Li, Be, and B, must be made by side
reactions and hence are not as prevalent. This is the primary reason
why oxygen is used for the energy-producing reactions of life on
Earth, even though fluorine is more electronegative; fluorine is
simply too rare to be depended on as a biological oxidant. If this
were not so, we probably would burn our foods with 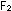 rather than
rather than 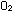 ,
and call the process fluoridation instead of oxidation. ,
and call the process fluoridation instead of oxidation.                   
The universe began as an unequal mixture of elements: few atoms
of high atomic number, fewer with odd atomic numbers, and almost
no Li, Be, or B.
|
|