|
Most of these antibiotics
are closed-ring compounds with many -O- or C=O groups, as in nonactin
(right).
Nonactin wraps around a potassium ion, with its oxygen atoms coordinated
to the ion, and with hydrophobic groups exposed to the exterior.
It is the exact opposite of the oildrop model of a protein, in which
the protein has a hydrophobic interior and a polar exterior. The
inverted structure of the antibiotic presumably makes it possible
for nonactin and a K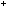 ion to diffuse through the lipid bilayer of the membrane.
ion to diffuse through the lipid bilayer of the membrane.
Nonactin, in effect, gives the ion a hydrophobic overcoat. Gramicidin
can carry all of the alkali metal ions through a membrane, but valinomycin
carries only K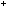 ,
Rb ,
Rb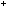 ,
or Cs ,
or Cs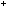 .
Such antibiotics are toxic because they make membranes susceptible
to alkali metal ions when they should not be. Cells waste their
ATP by pumping K .
Such antibiotics are toxic because they make membranes susceptible
to alkali metal ions when they should not be. Cells waste their
ATP by pumping K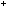 in and Na
in and Na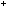 out, only to find them leaking the wrong way again, with the aid
of these carrier molecules.
out, only to find them leaking the wrong way again, with the aid
of these carrier molecules.
|
|