|
15. How does acid cause crystalline BeO to dissolve? How does base
produce a similar effect?
16. What is the formula of the beryllium ion in acid solution? What
is it in basic solution? What would happen to the ion if one began
with an acid solution of beryllium ions and slowly made it basic?
17. Why does fluorine not form an oxyion analogous to 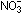 or
or 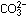 ? ?
18. What is the difference between a crystalline solid and a glass?
Which of the oxides of second-row elements forms a glass? How do
crystals and glasses differ in melting points?
19. Why is the carbon dioxide molecule linear, whereas the water
molecule is bent at an angle of 1050?
20. What is the dipole moment of the carbon dioxide molecule?
|
|
21. Write at least three possible bond models for the carbonate
ion. What would each of your models predict about the three carbonoxygen
bonds? How do the carbon-oxygen bond lengths in the real carbonate
ion compare with those in your model structures?
22. In ethylene, 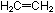 ,
all six atoms lie in a plane. Is this also true for hydrazine, ,
all six atoms lie in a plane. Is this also true for hydrazine, 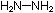 ?
Use VSEPR theory to sketch what the hydrazine molecule should look
like. ?
Use VSEPR theory to sketch what the hydrazine molecule should look
like.
23. In ethylene, the H-C-H bond angle at either end of the molecule
is 1200. Roughly what should the corresponding
H-N-H angle be in hydrazine?
24. What holds the ions together in a salt? What holds them together
in a metal?
25. Why will metals bend when pushed upon, whereas salts usually
crack? Explain in terms of ionic forces.
|