|
Even metallic sheen can be explained by structure. A glass surface
or a mirror appears bright because the light that falls on it is
reflected efficiently.
Mirrors are simply glass, with a thin metal layer as a backing.
When light strikes a metal, it is absorbed and the light energy
raises electrons to excited (higher) energy states.
There are many mobile electrons in a metal, and many closely spaced
energy levels. The excited electrons can move about, and can drop
back to their original low-energy states, giving back the energy
as photons of light.
Non-metals lack these closely spaced electronic energy states, so
light, once absorbed, is less likely to be reemitted.
Melting points are useful measures of the forces between molecules
or ions. Molecular solids, in which covalently bonded molecules
are packed together with nothing but van der Waals forces between
them, have low melting points.
|
|
Solid 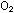 ,
for example, melts at -218°C. Lithium metal, which has mobile
electrons holding it together, melts at 179°C, and LiF, a typical
salt, melts at a much higher 842°C. ,
for example, melts at -218°C. Lithium metal, which has mobile
electrons holding it together, melts at 179°C, and LiF, a typical
salt, melts at a much higher 842°C.
Solids that are held together completely by covalent bonds in a
three-dimensional. network are the most tightly knit of all, and
carbon in the form of diamond has a melting point of over
3600°C.
|