|
BeO also is soluble in basic solution (right). The hydroxide ions
tear the BeO crystal apart by attacking 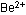 ions more strongly than water molecules can:
ions more strongly than water molecules can:
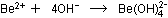
The oxide ions then are freed to interact with water molecules :
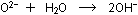
The overall reaction is the sum of the two:
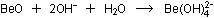
This is the species that was mentioned previously as being most
stable in basic solution, where a shortage of 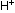 ions exists.
ions exists.
|
|
|