|
The lithium ions in 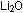 play a passive role, doing nothing more than counter-balancing the
negative charges of the
play a passive role, doing nothing more than counter-balancing the
negative charges of the 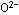 ions in the crystal, or the
ions in the crystal, or the 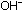 ions in the basic solution (see previous page).
ions in the basic solution (see previous page).  ions are hydrated by four water molecules, as mentioned previously.
ions are hydrated by four water molecules, as mentioned previously.
If we evaporate the basic solution obtained from 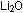 to dryness, crystals of lithium hydroxide (LiOH) are obtained, which
are made up of
to dryness, crystals of lithium hydroxide (LiOH) are obtained, which
are made up of  and
and 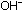 ions. Extra heating in a dry environment is required to drive water
molecules off and to reconvert the lithium hydroxide crystals to
lithium oxide:
ions. Extra heating in a dry environment is required to drive water
molecules off and to reconvert the lithium hydroxide crystals to
lithium oxide:

The behavior of lithium and its basic oxide that we have just seen
is typical of metals in general. Although lithium stands alone in
its basic behavior among the second-shell elements, metals and metal
hydroxides are very important among the heavier elements.
|
|
|